Abstract
Background: Patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) typically have poor treatment outcomes, especially patients who are ineligible for stem cell transplantation (SCT). Ibrutinib, a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase, is approved in the US for various B-cell malignancies. Preclinical data suggest potential synergy when combining ibrutinib with lenalidomide, a thalidomide analogue that disrupts signaling downstream of B-cell receptor and MYD88. An open-label, multicenter, phase 1b/2 study (NCT02077166) was initiated to evaluate the iR2 regimen of ibrutinib, lenalidomide, and rituximab in R/R DLBCL. Results from the ongoing phase 2 portion of the study evaluating the safety and activity of the iR2 regimen in SCT-ineligible adults aged ≥18 y with R/R non-germinal center B-cell-like (non-GCB) DLBCL per Hans method are presented here.
Methods: The iR2 regimen was administered at the recommended phase 2 dose (RP2D) of once-daily 560 mg PO ibrutinib with 20 mg PO lenalidomide on Days 1-21 and 375 mg/m2 IV rituximab on Day 1 of Cycles 1-6 in 28-day cycles (additional 25-mg cohort ongoing). The primary phase 2 efficacy endpoint was ORR; the null hypothesis of an ORR of 40% will be tested against the alternative hypothesis of an ORR >60%. Secondary endpoints included complete response (CR) rate, duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Response was based on investigator assessments and performed using CT or MRI scans after every 3 treatment cycles for the first 24 mo and every 6 mo thereafter. For rash and neutropenia, if clinically indicated, study medication was withheld until resolution or improvement to grade 1, and treatment with oral corticosteroids and antihistamines (for rash) or hematopoietic growth factors (for neutropenia) was initiated.
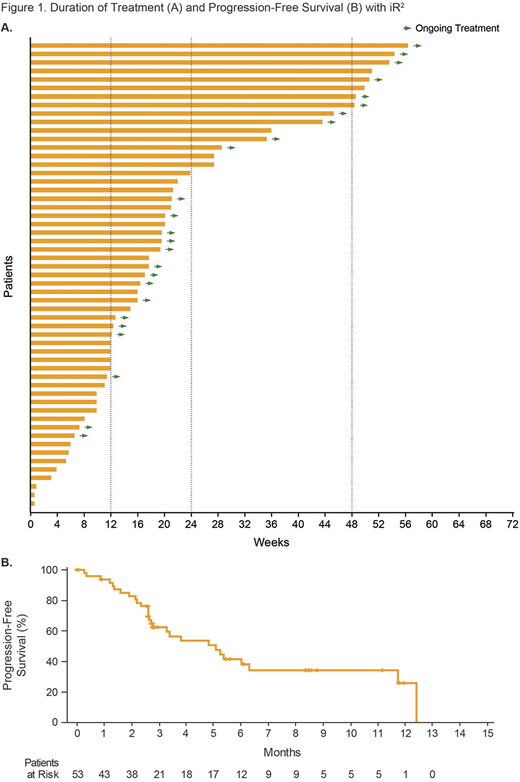
Results: A total of 55 patients were enrolled and treated at the RP2D with 20 mg PO lenalidomide in phase 2. The median age was 63 y; 58% were male; 64% had stage IV disease; 24% had primary refractory disease; 53% were refractory to the last therapy. Patients had received a median of 2 (range: 1-5) prior systemic therapies for DLBCL; the most common regimens were R-CHOP (71%), RICE (29%), and R-DHAP (16%). Among the 44 response-evaluable patients with follow-up imaging, the ORR was 55% (95% CI: 39%-70%; n=24) and included CR in 30% (n=13) and PR in 25% (n=11); 5 patients (11%) had stable disease. The median DOR was 9 mo for all responders and 10 mo for those who achieved a CR. The median maximum percent change from baseline in the size of the target lesion(s) was −61%. Among all 55 treated patients, the median duration of iR2 treatment was 4 mo (range: 0-13), and almost half (45%) of patients were still receiving iR2 treatment at the time of analysis (Figure 1A). Progressive disease was the most common reason for treatment discontinuation (45%). Median PFS was 5 mo (95% CI: 3-12; Figure 1B), with 6-mo and 12-mo PFS rates of 44% and 28%, respectively. Median OS was 17 mo (95% CI: 8-17), with 6-mo and 12-mo OS rates of 85% and 58%, respectively. For the 24 responders, median PFS was 12 mo (95% CI: 6-12), and median OS was 17 mo (95% CI: not evaluable). The majority (85%) of patients experienced a grade 3-4 TEAE; events reported in ≥5% of patients included neutropenia (33%), maculopapular rash (15%), anemia (11%), diarrhea, dyspnea, fatigue, and hypokalemia (5% each). Neutropenia, maculopapular rash, and anemia were the only grade 3-4 TEAEs in >2 patients considered related to either ibrutinib or lenalidomide. Of the 8 patients with grade 3/4 maculopapular rash, 7 received concomitant corticosteroids. Grade 5 TEAEs were experienced by 6 patients and included worsening of DLBCL (n=3), pneumonia (n=2), and sepsis (n=1). Doses of study treatment were temporarily interrupted or reduced due to TEAEs in 62% and 29% of patients, respectively. Discontinuation due to TEAEs occurred in 11% of patients (worsening of DLBCL [5%], pneumonia [4%], and sepsis [2%]). No cases of febrile neutropenia were reported.
Conclusions: The iR2 combination regimen of 560 mg ibrutinib, 20 mg lenalidomide, and 375 mg/m2 rituximab demonstrated promising activity with a manageable safety profile in these difficult-to-treat R/R non-GCB DLBCL patients ineligible for SCT. Evaluation of the iR2 regimen using a dose of 25 mg lenalidomide and biomarker analyses, including GEP, are ongoing.
Ramchandren:Merck: Research Funding; Bristol-Myers Squibb: Consultancy; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics LLC an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Johnson:Genmab: Consultancy; Novartis: Honoraria; Takeda: Honoraria, Travel, accommodations, expenses; Zenyaku Kogyo: Other: Travel, accommodations, expenses; Janssen: Consultancy, Research Funding; Kite: Consultancy; Incyte: Consultancy; Boeringher Ingelheim: Consultancy; Bristol-Myers Squibb: Honoraria; Epizyme: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Eisai: Research Funding. Ghosh:Forty seven Inc: Research Funding; SGN: Consultancy, Research Funding, Speakers Bureau; Juno: Consultancy, Research Funding; Celgene: Consultancy; Genentech: Research Funding; PCYC: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics, an Abbvie Company: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; TG Therapeutics: Honoraria, Research Funding; Spectrum: Consultancy; F. Hoffman-La Roche Ltd: Research Funding. Ardeshna:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses. Johnson:Takeda Pharma: Other: Funded an educational place for me to attend the Lugano Lymphoma conference in June 2017. Cunningham:Roche pharmaceuticals: Research Funding. Kassam:AbbVie: Equity Ownership. Radford:Pfizer: Research Funding; BMS: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Equity Ownership; GlaxoSmithKline: Equity Ownership; Celgene: Research Funding; Novartis: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Speakers Bureau. Bailly:Abbvie: Other: Travel, Accommodations, Expenses; Roche: Other: Travel, Accommodations, Expenses; Takeda: Other: Travel, Accommodations, Expenses. Munoz:Bayer: Consultancy, Speakers Bureau; Janssen: Consultancy; Kite Pharmaceuticals: Consultancy, Speakers Bureau; Juno: Consultancy; Bristol-Myers Squibb: Consultancy; Pfizer: Consultancy; Alexion: Consultancy; Pharmacyclics LLC, an ABBVIE Company: Consultancy. Ping:Pharmacyclics, an Abbvie company: Employment; Abbvie: Equity Ownership. Co:Abbvie: Employment, Equity Ownership, Other: Travel, Accommodations, Expenses. Neuenburg:Pharmacyclics, an Abbvie company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal